Polonij
84
Po
Skupina
16
Perioda
6
Blok
p
Protoni
Elektroni
Nevtroni
84
84
126
Splošne lastnosti
Vrstno število
84
Atomska teža
[210]
Masno število
210
Kategorija
Polkovine
Barva
Srebrna
Radioaktivno
Da
Named after Poland, native country of Madam Curie
Kristalna struktura
Primitivna kubična
Zgodovina
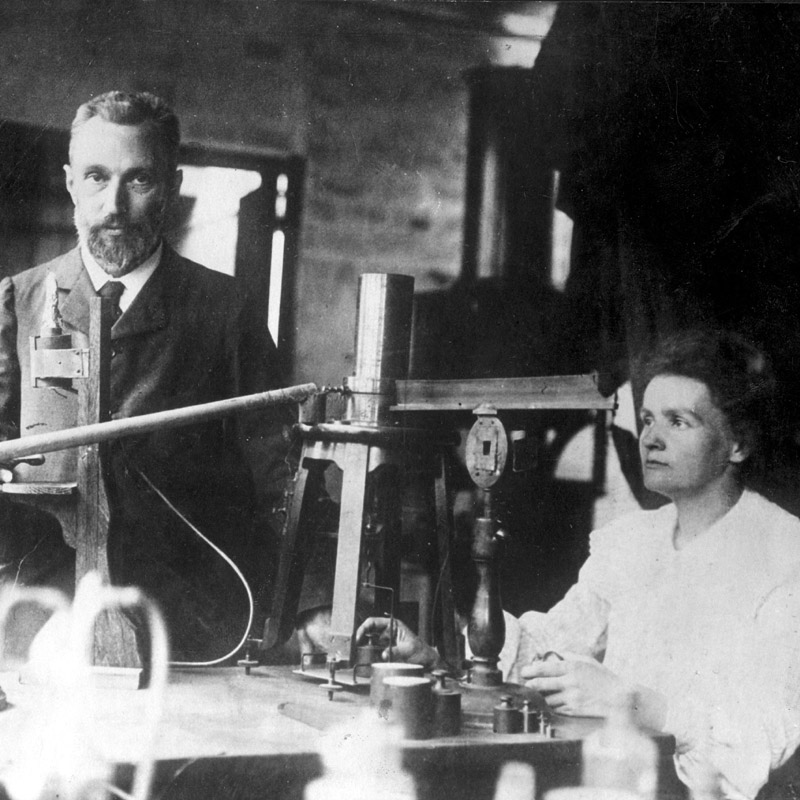
Polonium was discovered by Marie and Pierre Curie in 1898 in Paris.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity.
The dangers of working with radioactive elements were not known when the Curies made their discoveries.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity.
The dangers of working with radioactive elements were not known when the Curies made their discoveries.
Elektroni po lupinah
2, 8, 18, 32, 18, 6
Razporeditev elektronov
[Xe] 4f14 5d10 6s2 6p4
Polonium is obtained by irradiating bismuth with high-energy neutrons or protons
Fizikalne lastnosti
Faza snovi
Trdnina
Gostota
9,196 g/cm3
Tališče
527,15 K | 254 °C | 489,2 °F
Vrelišče
1235,15 K | 962 °C | 1763,6 °F
Talilna toplota
13 kJ/mol
Izparilna toplota
100 kJ/mol
Toplotna kapaciteta
- J/g·K
Zastopanost v Zemljini skorji
n/p
Zastopanost v vesolju
n/p
CAS številka
7440-08-6
PubChem CID številka
n/p
Atomske lastnosti
Atomski polmer
168 pm
Kovalentni polmer
140 pm
Elektronegativnost
2,00 (Paulingova lestvica)
Ionizacijski potencial
8,417 eV
Atomski volumen
22,23 cm3/mol
Toplotna prevodnost
0,2 W/cm·K
Oksidacijska stanja
-2, 2, 4, 6
Uporabe
Polonium is used to eliminate static electricity produced during processes such as rolling paper, wire and sheet metal.
Polonium can be mixed or alloyed with beryllium to provide a source of neutrons.
It is also used in anti-static brushes to eliminate dust on photographic film.
Polonium can be mixed or alloyed with beryllium to provide a source of neutrons.
It is also used in anti-static brushes to eliminate dust on photographic film.
Polonium is highly dangerous and radioactive
Izotopi
Stabilni izotopi
-Nestabilni izotopi
188Po, 189Po, 190Po, 191Po, 192Po, 193Po, 194Po, 195Po, 196Po, 197Po, 198Po, 199Po, 200Po, 201Po, 202Po, 203Po, 204Po, 205Po, 206Po, 207Po, 208Po, 209Po, 210Po, 211Po, 212Po, 213Po, 214Po, 215Po, 216Po, 217Po, 218Po, 219Po, 220Po